Little Hearts in Focus: Uncovering the Genetic Code Behind Congenital Heart Defects in Children
A study by IIMCB scientists published in BMC Genomics uses computational biology to reveal regions of DNA that could influence improper heart development. This significant global health issue affects over one million families worldwide each year, and the research was sparked by a deeply personal experience.
Each year, more than 1.3 million families around the world welcome a child born with a heart that did not develop properly [1]. Tragically, roughly 180,000 of those babies do not live to see early childhood [1,2]. Congenital heart disease (CHD) – a condition that affects the heart’s normal structure and function – remains one of the most common birth defects and continues to raise fundamental questions about its underlying causes.
 Research team from the Laboratory of Zebrafish Developmental Genomics at IIMCB: od lewej Costantino Parisi, dr Cecilia Lanny Winata, dr Shikha Vashisht
Research team from the Laboratory of Zebrafish Developmental Genomics at IIMCB: od lewej Costantino Parisi, dr Cecilia Lanny Winata, dr Shikha Vashisht
While many cases are linked to genetic factors, most studies have focused on changes in genes that produce proteins. But these genes make up just 1% of our DNA. The remaining 99% – the non-coding genome – harbours the majority of genetic variants linked to CHD, and their mechanisms by which they contribute to the condition has long been considered a mystery.
Researchers from the Laboratory of Zebrafish Developmental Genomics at the International Institute of Molecular and Cell Biology (IIMCB) decided to look deeper into this unexplored territory. Their new findings, published in BMC Genomics, reveal specific non-coding regions that may play a role in the development of CHD, pointing to previously unknown mechanisms that help shape the human heart.
KEY FINDINGS OF THE TEAM OF DR. CECILIA LANNY WINATA
• We discovered of over 2,000 genetic "switches" that may cause congenital heart disease. These genetic changes may disrupt critical heart development signals.
• 63 genetic variations directly affect gene activity in heart tissue.
• Specific genetic change in the genes MYBPC3 and ACTC1 may weaken crucial heart protein interaction, potentially affecting normal heart muscle function and development.
At the Heart of the Problem
For Shikha Vashisht, first author of the study, the project was driven by more than scientific curiosity. Years ago, her niece was born with a serious defect that required an open-heart surgery shortly after birth – an experience that left a lasting impression.
“Standing in that hospital, watching such a tiny baby going through a major procedure, I had to ask: how does a baby's heart, which should develop normally during pregnancy, end up with such a critical life-threatening defect, and is there a way to prevent or cure this condition?” Vashisht recalls. “This experience opened my eyes to the countless families facing similar situations. It was not just about understanding the science anymore - it became about finding answers for real families living through these challenging moments.”
Looking Beyond the Obvious
Using advanced computational methods combined with publicly available genetic data, the team identified both protein-coding and non-coding DNA variants that may be linked to CHD. In particular, they found more than 2,000 potential heart-specific enhancers – short, non-coding DNA sequences that help regulate gene activation. Many of the detected genomic regions were evolutionarily conserved and contained binding sites for key transcription factors involved in heart development. The researchers also discovered a potentially harmful mutation in a protein-coding gene, MYBPC3, which may disrupt interactions with other heart-specific proteins and impair normal heart function.
“This research highlights how subtle changes, like single mutations, can compromise their ability to direct heart development, leading to congenital heart defects.” adds Dr. Cecilia Winata, head of the Laboratory of Zebrafish Developmental Genomics at IIMCB and a corresponding author of the article in BMC Genomics.
While the study is based on computational analysis, it provides a valuable foundation for future experimental research. The team is now working on validating the identified elements in the lab. If confirmed, these results could help explain how changes in the genome’s regulatory system contribute to CHD – and why some children are born with heart defects despite having no mutations in protein-coding genes.
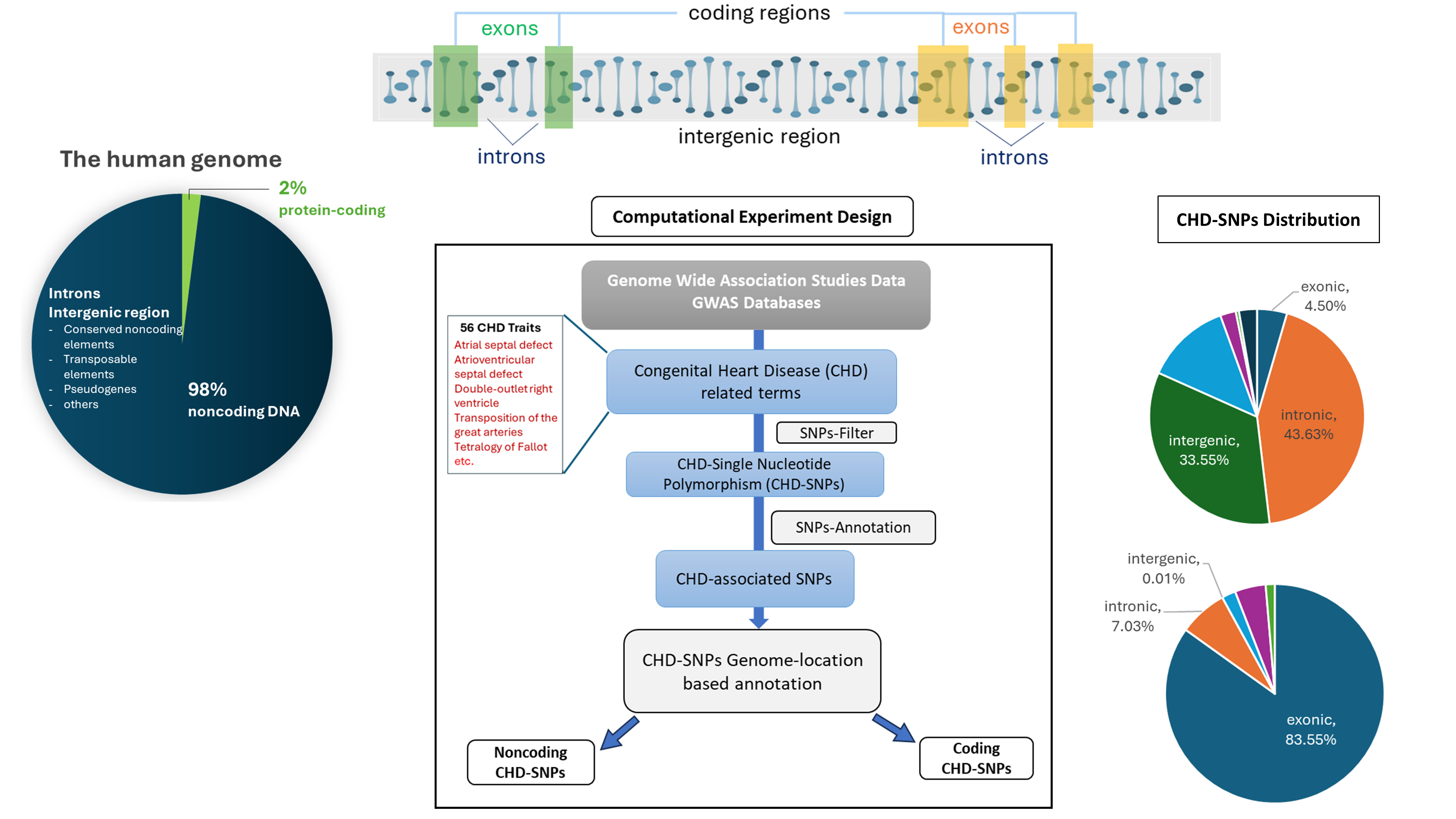 Computational pipeline used to discover genetic variants associated congenital heart diseases.
Computational pipeline used to discover genetic variants associated congenital heart diseases.
The large majority of human genome consists mostly of noncoding elements - those that do not code for proteins.
A genetic variant located in such regions could result in disease by disrupting critical heart development signals.
“We have been able to show that the noncoding regions of the genome which is often considered as ‘junk DNA’, actually contained many essential elements that are associated with various forms of CHD. This work emphasizes the importance of further exploration of the noncoding genome.” says Shikha Vashisht.
A Step Towards Better Understanding of CHD
The study provides a systematic approach to understanding the genetic basis of Congenital Heart Disease, highlighting the complex role of both coding and noncoding genetic variants in heart development. The study’s results may be of interest to researchers working on the genetics of complex diseases, as well as to clinicians and genetic counselors.
“This research is not about finding a single cause,” adds dr. Vashisht. “It’s about piecing together the complex genetic puzzle behind CHD. We hope that one day this knowledge will support better diagnosis and eventually improve care for children and families affected by this condition. A personal experience with my niece continues to drive my dedication to understanding the genetic foundations of CHD”, shares the IIMCB researcher.
“It is important to emphasize that this basic research is an important step toward a better understanding of the complex roots of CHD. While it is not intended as as a direct therapeutic or diagnostic development, it lays the foundation for future advances in those areas” adds Dr. Cecilia Lanny Winata.
The full study is available open access at:
https://doi.org/10.1186/s12864-025-11232-6
[1] Liu Y, Chen S, Zühlke L, et al. Global, regional, and national burden of congenital heart disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Child Adolesc Health. 2019;3(12):855–870. doi:10.1016/S2352-4642(19)30402-X
[2] World Health Organization. Birth defects, 2022. https://www.who.int/news-room/fact-sheets/detail/birth-defects
This work was supported by the National Science Center, Poland, under research projects no 2018/29/B/NZ2/01010, 2019/35/B/NZ2/02548 and 2022/47/B/NZ2/02926.

