Bacteria use a wide range of defense strategies to prevent viral infection or replication of the viruses. One of them is abortive infection (Abi) which is an altruistic process of programmed death of the infected cell to prevent the virus from replicating and spreading to other cells. A variety of Abi systems has been identified that are predicted to work through very different mechanisms. AbiK and Abi-P2 proteins are abortive infection effector proteins that are related to reverse transcriptases (RTs) – enzymes which synthesize DNA using RNA as a template. Previous biochemical studies of AbiK have shown that this enzyme can synthesize DNA without any template. Moreover, it does not require a short DNA fragment to start nucleic acid synthesis. Instead it attaches the first nucleotide of the growing DNA chain to one of its own residues through a mechanism called protein-priming.
The scientists from the Laboratory of Protein Structure determined the first structures of DNA polymerases AbiK and Abi-P2 using X-ray crystallography and cryoelectron microscopy. Both proteins have two parts: a catalytic RT-like domain and a domain unique for Abi proteins which is made of α-helices and serves to stabilize the nascent DNA chain. The structures revealed that AbiK and Abi-P2 form hexamers and trimers, respectively, which is unprecedented for DNA polymerases. The structure of wild-type AbiK contained a single-stranded DNA chain that was covalently linked to the priming residue with its 5′ end whereas its 3′ portion was accommodated in the central channel of the enzyme. Additionally, a cryo-EM structure was determined for an AbiK variant that contained a substitution of the protein-priming residue and thus did not contain any covalently bound DNA. Comparison of the structures with and without DNA showed that protein priming by AbiK involves a change in the arrangement of a mobile part of the protein harboring the amino acid residue involved in priming.
The work has been published in Nucleic Acids Research.
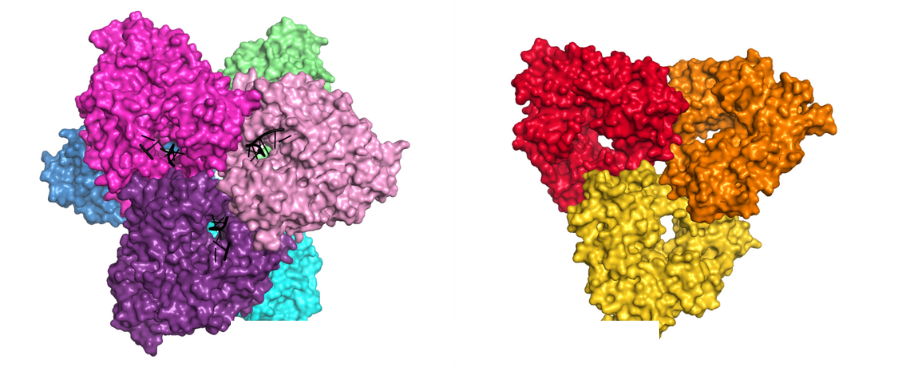
Crystal structures of AbiK-DNA adduct (left) and Abi-P2 (right) polymerases shown in surface representation.
