Catalytic triads are among the most common enzyme active sites. They are composed of three residues: a residue that performs the nucleophilic attack on a substrate and thus launches the reaction (typically a serine or cysteine), a histidine that absorbs a proton from the nucleophile and activates it, and a third residue, typically with an acidic or amide side chain, that stabilizes the histidine. Triads can be divided into Nε and Nδ-configured ones depending on which histidine nitrogen is directed towards the nucleophile. The two configurations seem equivalent, but they are distinguishable due to a mildly higher basicity of the Nε atom, which makes the Nδ configuration more stable. As a result, this configuration prevails for non-catalytic triads, free of additional constraints. In catalytic triads the histidine needs to fulfill its duty and thus the situation depends on the type of the nucleophile. In cysteine triads, the abstraction of the proton from the nucleophile is easier and the transfer occurs prior to the catalysis. Thus, the higher basicity of the nitrogen directed towards the nucleophile is not essential. As a consequence, the cysteine catalytic triads follow the trend observed for non-catalytic triads and are predominantly Nδ-configured. In contrast, in serine triads, the proton transfer is difficult. It is only possible during the reaction thanks to the additional acidifying influence of the substrate. Thus, the basicity of the histidine nitrogen directed towards the nucleophile is of key importance. This results in almost exclusive presence of the less stable (frustrated) Nε-configured catalytic triads among serine nucleophile employing enzymes including peptidases, esterases, transferases, oxidoreductases and lyases.
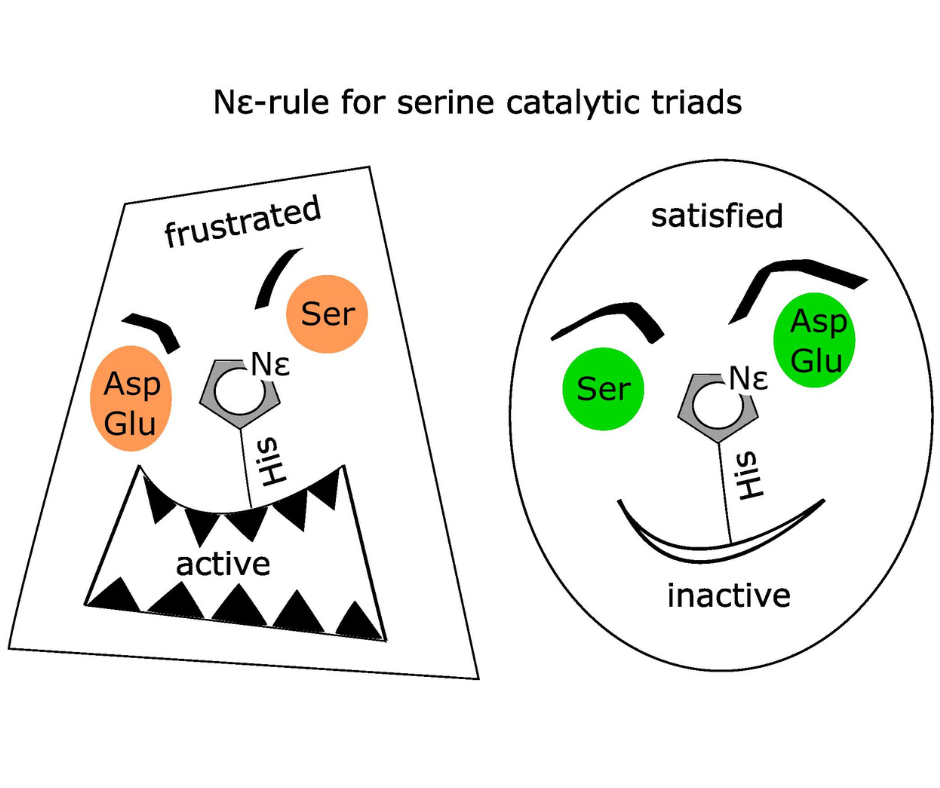
Nε-rule for serine, but not cysteine catalytic triads: Serine catalytic triads come in two varieties, with the Nε of the histidine pointing towards the serine (left) or towards the third triad residue (right). The first arrangement is less stable (“frustrated”), but better supports the otherwise difficult proton transfer from the serine during catalysis. Therefore, it is found in all serine catalytic triads (except CheB and PGM), whereas the Nδ-arrangement predominates in non-catalytic triads.
