G3BP proteins anchor the TSC complex to lysosomes and thus inhibit the metabolic driver MTOR - a signaling protein that plays a central role in tuberous sclerosis and its treatment. This is reported in the renowned journal Cell. The study was led by Kathrin Thedieck and Christiane Opitz together with a Europe-wide research network, including researchers from the International Institute of Molecular and Cell Biology in Warsaw.
The signaling protein MTOR (Mechanistic Target of Rapamycin) is a sensor for nutrients such as amino acids and sugars, and extracellular signals including trophic factors or neurotransmitters. When sufficient nutrients are available, MTOR, in response to extracellular stimuli, boosts metabolism and ensures that sufficient energy and building blocks are available for the growth and function of all cells in the human body. Not surprisingly, inaccuracies in its activation lead to serious diseases, many of which have neurological symptoms. Dysregulated MTOR in the brain causes anatomical malformations as well as changes in brain cell activity that eventually often results in epilepsy and, in some cases, also in neuropsychiatric problems.
To prevent errors in MTOR-based signal processing, the cell controls its activity very precisely. This is achieved through so-called suppressors, molecules that inhibit a protein and help to regulate its activity. The TSC complex is such a suppressor for MTOR. It is named after the disease that is caused by its absence - tuberous sclerosis complex (TSC) disease. Together with MTOR, the TSC complex localizes to small cellular structures, the lysosomes, where it keeps MTOR in check. If the TSC complex - for example due to changes (mutations) in one of its components - no longer remains at the lysosome, this can lead to excessive MTOR activity with severe consequences for human health.
The G3BP proteins: molecular TSC anchors at lysosomes that regulate brain activity
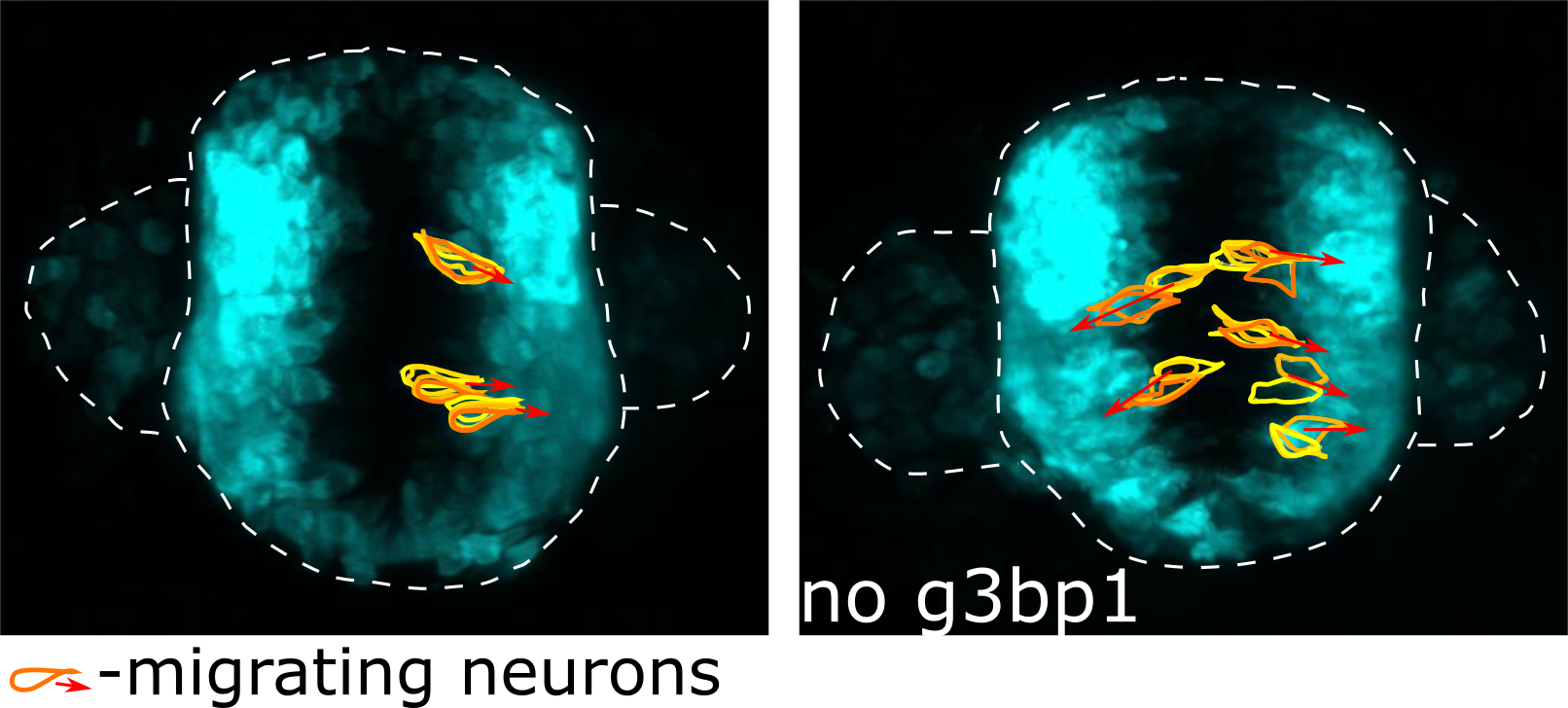
The teams led by Kathrin Thedieck and Christiane Opitz therefore investigated how the TSC complex binds to lysosomes. They discovered that the G3BP (Ras GTPase-activating protein-binding protein) proteins localize to lysosomes, together with the TSC complex. There, the G3BP proteins form an anchor that ensures that the TSC complex can bind to the lysosomes. This anchor function plays a crucial role, for instance in breast cancer. If the amount of G3BP decreases, not only MTOR activity but also cell motility is increased in cancer cell cultures. But G3BP proteins also inhibit MTOR in the brain. Two groups, led by Peter de Witte from Leuven and by Jacek Jaworski from the IIMCB joint forces to analyze G3BP’s role in the brain, using zebrafish, an important animal model for pharmaceutical research. Consequently, researchers from Leuven and Warsaw, including Justyna Zmorzynska and Magdalena Kedra from the Laboratory of Molecular and Cellular Neurobiology (IIMCB), showed that loss of G3BP led to disturbances in brain development, neuronal hyperactivity and behavioral abnormalities reminiscent of epilepsy in humans. Importantly, compounds that target MTOR suppressed the neuronal hyperactivity. Therefore, the scientists anticipate that patients with neurological disorders in which the G3BP proteins are defective could benefit from MTOR inhibitors.
G3BPs tether the TSC complex to lysosomes and suppress mTORC1 signaling. Prentzell MT*, Rehbein U*, Cadena Sandoval M*, De Meulemeester A*, Baumeister R, Brohée L, Berdel B, Bockwoldt M, Carroll B, Chowdhury SR, von Deimling A, Demetriades C, Figlia G, Genomics England Research Consortium, Eca Guimaraes de Araujo M, Heberle AM, Heiland I, Holzwarth B, Huber LA, Jaworski J, Kedra M, Kern K, Kopach A, Korolchuk VI, van 't Land-Kuper I, Macias M, Nellist M, Palm W, Pusch S, Ramos Pittol JM, Reil M, Reintjes A, Reuter F, Sampson JR, Scheldeman C, Siekierska A, Stefan E, Teleman AA, Thomas LE, Torres-Quesada O, Trump S, West HD, de Witte P, Woltering S, Yordanov T, Zmorzynska J, Opitz CA#, Thedieck K#. Cell 2021
DOI: https://doi.org/10.1016/j.cell.2020.12.024