
Each year, International Institute of Molecular and Cell Biology provides wide range of short term summer training programs (1-4 months) for BSc and MSc students.
For additional information, please check scientific profile of our laboratories and contact Lab Leaders directly: Laboratories

Determination of the size and shape of the macromolecules in solution

The ProteomeLab XL-A/XL-I in-solution characterization of proteins, oligomers, aggregates, particles, colloids, and small structures delivers accurate results you can rely on time and again. In-solution characterization allows for testing in native conditions, meaning you determine the sample testing environment that best suits your needs. The unique column-free separating technique of the ProteomeLab XL-A/XL-I measures the relative change in the distribution of molecular weights, providing an efficient way to measure heterogeneity, stoichiometry and self-associating systems. And, because the measurements are based on the first principles of thermodynamics and hydrodynamics, no standards or calibrations are required. As a result, you spend less time on setup and more time on discovery. From: Beckman-Coulter web page. https://www.beckmancoulter.com. Contact: Roman Szczepanowski, This email address is being protected from spambots. You need JavaScript enabled to view it.
Characterization of molecular interactions of macromolecules with ligands or other biomolecules

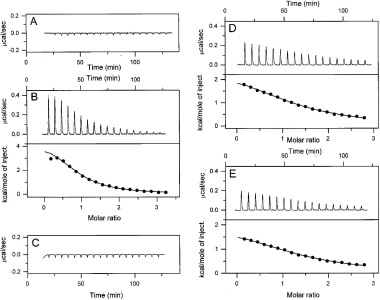
The VP-ITC system is an ultrasensitive isothermal titration calorimeter designed for ease-of-use. All functions are operated through software to minimize operator involvement and facilitate fast and accurate analyses without the need for expertise in thermodynamics. The capabilities of ITC as a technique, combined with the unique performance features of the VP-ITC have made this system the best selling ultrasensitive isothermal titration calorimeter in the world. Applications include: Characterization of molecular interactions of small molecules, proteins, antibodies, nucleic acids, lipids and other biomolecules. Lead optimization. Enzyme kinetics. Assessment of the effect of molecular structure changes on binding mechanisms. Assessment of biological activity.
Why VP-ITC?
More than just affinities: Simultaneous determination of all binding parameters in a single experiment - information unobtainable from more limited binding assays. Directly measure sub-millimolar to nanomolar binding constants (102 to 109 M-1). Measure nanomolar to picomolar binding constants (109 to 1012 M-1) using the competitive binding technique. Application versatility: Investigate any biomolecular interaction with high sensitivity. True in-solution technique: No labeling or immobilization required. No buffer restrictions. Easily handles turbid solutions. Easy to use: Unattended operation after sample loading. Complete system: No additional accessories to purchase. No reagents are required. Info from: VP-DSC Microcal web page www.microcalorimetry.com. Contact: Roman Szczepanowski, This email address is being protected from spambots. You need JavaScript enabled to view it.
Determination of the thermodynamic parameters of the macromolecules in solution

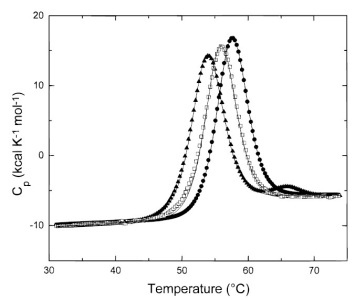
The VP-DSC is a differential scanning microcalorimeter. The reference and sample cells are fixed in place lollipop shaped vessels with effective volumes of approximately 0.5 ml. The cells are constructed from a tantalum alloy and protrude out of the adiabatic chamber via their 1.5 mm (inner diameter) access tubes. The approximate volume of each cell stem is 0.085 ml.
The cell material (Tantaloy 61™) has chemical properties quite similar to glass. This alloy is immune to attack by almost all acids, having been tested under widely varying concentrations/temperatures involving chlorine, bromine, hydrochloric acid, nitric acid and sulfuric acid. Please refer to the dedicated manual outlining the chemical properties of Tantaloy 61™ for more detailed information.
The VP-DSC operates in the temperature range of -10° to 130° Celsius. It is capable of both upscanning and downscanning with no external heating/cooling source. Scanrates are user selectable and fall in the range of 0°/hr to +90°/hr in the upscan mode and 0°/hr to -60°/hr in the downscan mode. The VP-DSC can also be used at constant temperature for long periods of time (Isoscan) for shelf life studies and/or for evaluating the stability of drug or other chemical formulations. The duration of such a study is limited only by the available space on the PC hard disk.
For scanning solutions above their boiling point, the VP-DSC has a self-contained pressurizing system allowing positive pressures from 0 - 30 p.s.i. Applications requiring higher pressures can be conducted with the addition of an external pressure source and accessory pressure cap, available from MicroCal. Pressure applied to the VP-DSC cells should never exceed 80 p.s.i. or irreversible damage to the cells may be incurred!
Temperature differences between the reference cell and the sample cell are measured, calibrated to power units and displayed to the user as well as saved to disk. This data channel is referred to as the DP signal, or the differential power between the reference cell and the sample cell. Calibration of this signal is obtained electrically by administering a known quantity of power through a resistive heater element located on the sample cell.
In general, an exothermic (heat is released) event in the sample cell will cause the DP signal to deflect in the negative direction. Likewise, an endothermic (heat is absorbed) event in the sample cell will cause the DP signal to deflect in the positive direction. From: VP-DSC MicroCalorimeter User's Manual, MicroCal. www.microcalorimetry.com Contact: Roman Szczepanowski, This email address is being protected from spambots. You need JavaScript enabled to view it.

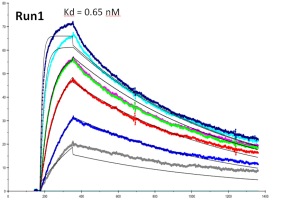
Biacore 3000 is the highest performance research system available for label free studies of biomolecular binding. Samples ranging from small molecules to crude extracts, lipid vesicles, viruses, bacteria and eucaryotic cells can be studied. Biacore 3000 answers questions about the speed, strength and specificity of binding and determines the active concentrations of components. Technical innovations meet the highest demands for sensitivity, efficiency and flexibility.With new wizard driven and automated recovery functions including the novel Surface Prep unit for increased capacity recovery, Biacore 3000 is an ideal tool for functional proteomics. Increased integration with mass spectrometry characterization is provided by the enhanced recovery and ability to directly deliver sample to a MALDI target as well as delivery of digestion enzymes and MALDI matrix, all within the autosampler of the system. The knowledge and experience of Biacore has been incorporated into Wizards which guide the user without effort through preparation, experimentation and evaluation. Biacore Wizards provide a trend analysis and preliminary results at the end of runs. Conditional IF/THEN statements ensure that Biacore 3000 responds correctly to changes in run conditions.
The most advanced software available enables detailed evaluation of results. Biacore 3000 is designed for individual sample characterization where the highest resolution in kinetic analysis is essential and for automation of multi-sample analyses. From: Biacore 3000 Product Information Sheet. Contact: Krzysztof Skowronek, This email address is being protected from spambots. You need JavaScript enabled to view it.

Circular Dichroism (CD) relies on the differential absorption of left and right circularly polarised radiation by chromophores which either possess intrinsic chirality or are placed in chiral environments. Proteins possess a number of chromophores which can give rise to CD signals. In the far UV region (240-180 nm), which corresponds to peptide bond absorption, the CD spectrum can be analysed to give the content of regular secondary structural features such as a-helix and b-sheet. The CD spectrum in the near UV region (320-260 nm) reflects the environments of the aromatic amino acid side chains and thus gives information about the tertiary structure of the protein. Other non-protein chromophores such as flavin and haem moieties can give rise to CD signals which depend on the precise environment of the chromophore concerned. Because of its relatively modest resource demands, CD has been used extensively to give useful information about protein structure, the extent and rate of structural changes and ligand binding. In the protein design field, CD is used to assess the structure and stability of the designed protein fragments. Studies of protein folding make extensive use of CD to examine the folding pathway the technique has been especially important in characterising molten globule intermediates which may be involved in the folding process. CD is an extremely useful technique for assessing the structural integrity of membrane proteins during extraction and characterisation procedures. The interactions between chromophores can give rise to characteristic CD signals. This is well illustrated by the case of the light harvesting complex from photosynthetic bacteria, where the CD spectra can be analysed to indicate the extent of orbital overlap between the rings of bacteriochlorophyll molecules. It is therefore evident that CD is a versatile technique in structural biology, with an increasingly wide range of applications. From: Sharon M. Kelly, Nicholas C. Price. The Use of Circular Dichroism in the Investigation of Protein Structure and Function. Current Protein & Peptide Science. Contact: Roman Szczepanowski, This email address is being protected from spambots. You need JavaScript enabled to view it. or Krzysztof Skowronek, This email address is being protected from spambots. You need JavaScript enabled to view it.

Fluorescence spectrometry can be as much as thousands of times more sensitive than absorbence techniques. This makes it possible to analyze samples in the nanogram to picogram range.
Fluorescence is used to identify or quantify specific molecules in complex mixtures. If the compound of interest is not fluorescent by itself, it can be labeled with specific fluorescent probes. The RF-5301PC‘s synchronous scanning mode allows mixtures of fluorochromes to be analyzed. Biological activity of enzymes can be assayed using substrates that yield a fluorescent product after cleavage.
The RF-5301PC is computer driven for data acquisition and processing. Its high throughput optical system in the RF-5301PC employs a blazed holographic grating, photomultiplier and digital signal processing to provide an excellent signal:noise ratio. From: Shimadzu Product Information Sheet. Contact: Roman Szczepanowski, This email address is being protected from spambots. You need JavaScript enabled to view it.
