Our goal is to understand how nerve cells develop their characteristic shape, critical for the functioning of the brain. Our "gateway" to the world of neurons is the protein mTOR. By utilizing molecular and cellular biology tools, we have come to understand what functions of mTOR are essential for neurons. We want to use this knowledge to diagnose and treat tuberous sclerosis, a disease caused by excessive mTOR activity.
Research Summary
Since the inception of our laboratory, our goal has been to understand how neurons develop their characteristic shape, which is crucial for brain function. We have sought to identify such mechanisms by studying the mammalian/mechanistic target of rapamycin (mTOR) protein, which is involved in neuronal development and various neuropathologies known as mTORopathies. mTOR is a serine-threonine kinase involved in almost all aspects of mammalian cell function. It forms two protein complexes that were originally thought to regulate translation (mTORC1) or affect the actin cytoskeleton (mTORC2). My postdoctoral work showed that regulating mTOR-dependent translation contributes to dendritogenesis. However, our subsequent work has shown that mTOR functions during neuronal development go beyond the canonical control of translation, e.g., cytoskeleton regulation, intracellular transport or transcription. Our recent studies focus mainly on mTOR functions in neuronal nuclei. An essential part of our work has been to apply our findings in cellular models to clinically relevant models and material. We focused on tuberous sclerosis (TSC), a disease caused by hyperactive mTOR. As a result, we have identified new potential drug targets (TrkB, GCLC) and predictive biomarkers for epilepsy in TSC. In parallel, in recent years we have investigated the mechanisms underlying the stability of neuronal morphology associated with the development of depression.
Scientific Impact
- Discovery of numerous regulators of neuronal cell morphology, including several mTOR substrates.
- Identification of GCLC and TrkB as new potential therapeutic targets in tuberous sclerosis.
- Development of predictive biomarkers for drug-resistant epilepsy in children with tuberous sclerosis.
Future Goals
Our primary goal for the coming years is to find out what functions mTOR fulfills in the nucleus of neurons and how they affect their proper development and function. Our current research suggests that destabilizing nuclear mTOR functions, such as controlling transcription factors, can lead to epilepsy. Therefore, we want to test this hypothesis thoroughly.
Collaborations
Our key collaborators include Prof. Kathrin Thedieck, Prof. Sergiusz Jóźwiak, Prof. Katarzyna Kotulska, Prof. David Kwiatkowski, Prof. Eleonora Aronica, Prof. Leszek Kaczmarek and Prof. Ewelina Knapska. We are working to understand how molecular biology translates into clinical features of tuberous sclerosis or depression and how this knowledge can be used to help patients.
Comment
"Our research focuses on the molecular basis of the development and stability of neuronal networks. In particular, we focus on the molecular functions of the mTOR protein, which will enable better diagnosis and potential therapy of mTORopathies, diseases caused by mTOR hyperactivity", says Prof. Jacek Jaworski.
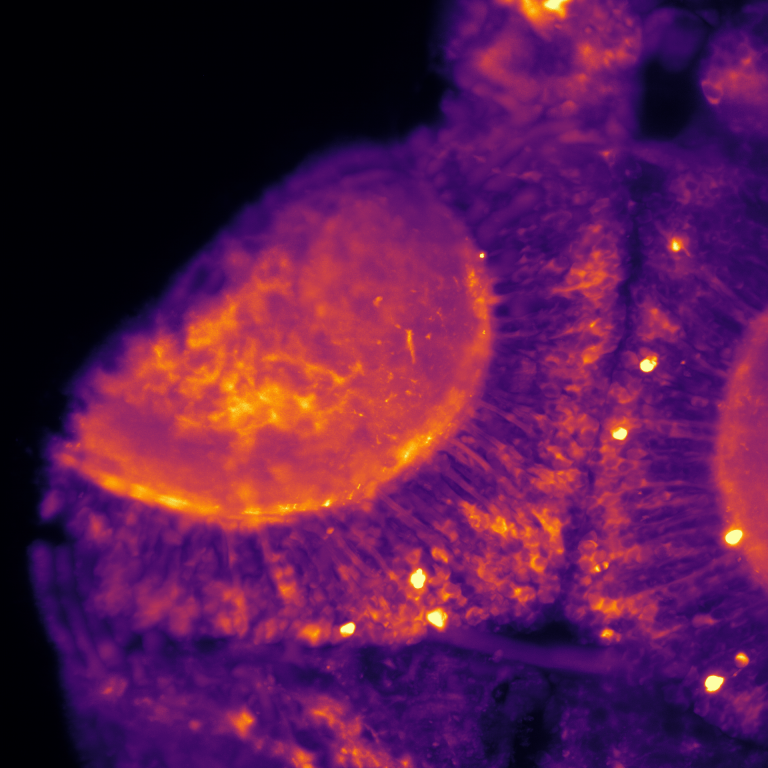
Activity of neurons in zebrafish brain measured using genetic calcium ion sensors after inhibition of chromatin modifying enzymes. Brighter color represents higher activity. Illustration by Roberto Pagano
 |
Jacek Jaworski, PhD, Professor
Correspondence address:
Laboratory of Molecular and Cellular Neurobiology
International Institute of Molecular and Cell Biology
4 Ks. Trojdena Street, 02-109 Warsaw, Poland
Email: This email address is being protected from spambots. You need JavaScript enabled to view it.
tel: +48 (22) 597 0755; fax: +48 (22) 597 0715
|
DEGREES
2014 - Professor of Biological Sciences, nomination by the President of the Republic of Poland
2010 - DSc Habil in Molecular Biology, Warsaw University, Poland
2001 - PhD in Molecular Neurobiology, Nencki Institute of Experimental Biology, Polish Academy of Sciences, Warsaw, Poland
1996 - MSc in Biology, Department of Genetics, Warsaw University, Poland
PROFESSIONAL EXPERIENCE
2018-present - Deputy Director for Science, International Institute of Molecular and Cell Biology in Warsaw, Poland
2010 - 2013 - Deputy Director, International Institute of Molecular and Cell Biology in Warsaw, Poland
2005-present - Professor, Head of Laboratory of Molecular and Cellular Neurobiology, International Institute of Molecular and Cell Biology in Warsaw, Poland
RESEARCH TRAINING
2016 - Research visit (3 weeks) with Prof. William Harris, Cambridge University, Cambridge, UK
2011 - Research visit (2 weeks) with Dr. Carlo Sala, CNR Institute of Neuroscience and Instituto Neurologico Carlo Besta, Milan, Italy
2006 - Research visit (1 month) with Dr. C.C. Hoogenraad, Erasmus Medical Center, Rotterdam, Holland
2002-2005 - Postdoctoral Associate with Prof. Morgan Sheng, Picower Center for Learning and Memory, Massachusetts Institute of Technology and Howard Hughes Medical Institute, Cambridge, MA, USA
2000 - Research training with Dr. J. Guzowski, ARL Division of Neural Systems, Memory and Aging, University of Arizona, Tucson, USA
1997-2001 - Research training (7 months) with Prof. J. Mallet, Laboratoire de Genetique Moleculaire de la Neurotransmission et des Processus Neurodegeneratifs (LGN), UMR 9923 CNRS, Paris, France
1996-2002 - PhD student (until 2001) and Postdoctoral Associate (until May 2002) with Prof. L. Kaczmarek, Laboratory of Molecular Neurobiology, Nencki Institute of Experimental Biology, Polish Academy of Sciences, Warsaw, Poland
1995-1996 - Master’s degree, Prof. P. Węgleński, Department of Genetics, Warsaw University, Poland
FELLOWSHIPS AND AWARDS
2018 - TEAM, Foundation for Polish Science
2014 - Master Award, Foundation for Polish Science
2011 - Prime Minister Award for habilitation thesis
2009 - 2nd Division (Biological Sciences) of Polish Academy of Sciences Award for series of publications on MMP9 (together with teams of Prof. Kaczmarek and Dr. Wilczynski)
2005 - Konorski Award for best publication of 2004 in the field of neuroscience (Kowalczyk et al., JCB, 2004, 167:209-213), Polish Neuroscience Society and Polish Academy of Sciences
2002 - Prime Minister Award for PhD thesis
2001 - Foundation for Polish Science National Scholarship for Young Investigators (1 year scholarship)
2000 - EMBO Short Term Fellowship
1999 - Polish Network for Cell and Molecular Biology UNESCO/PAN Scholarship
1997 - Bourse de Stage du Gouvernement Francaise (French Government Scholarship)
MEMBERSHIP IN SCIENTIFIC SOCIETIES, ORGANIZATIONS, AND PANELS
2019 - Member, Scientific Advisory Board of the Institute of Pharmacology, Polish Academy of Sciences
2017 - Vice President, Polish Neuroscience Society (term 2017-2019)
2015 - Corresponding Member, Warsaw Scientific Society
2015 - Member, Scientific Advisory Board of the Nencki Institute of Experimental Biology, Polish Academy of Sciences
2011 - Member, Neurobiology Committee, Polish Academy of Sciences (terms 2011-2014; 2015-2018; 2019-2020)
DOCTORATES DEFENDED UNDER LAB LEADER’S SUPERVISION
Ł. Świech, A. Malik, M. Perycz, M. Urbańska, A. Skałecka, J. Lipka, A. Urbańska, M. Firkowska, K. Kisielewska, A. Kościelny.

Lab Leader:
Senior Researchers:
-
Ewa Liszewska, PhD
-
Małgorzata Urbańska, PhD
Postdoctoral Researchers:
Research Specialist:
-
Katarzyna Machnicka, MSc
-
Katarzyna Durczyńska, MSc
-
Karolina Protokowicz, MSc
PhD Students:
-
Olga Doszyń, MSc
-
Shiwani Kumari, MSc
-
Katarzyna Orzoł, MSc
Lab Technician:
Laboratory Support Specialist:
Volunteers:
-
Julia Łukasiewicz
-
Gabriela Chmurzyńska
-
Wiktoria Kowalska
-
Antonina Ożyńska




