Our laboratory investigates how cells maintain the health of their proteome – a process known as proteostasis. We are particularly interested in the spatial and temporal regulation of protein degradation pathways in mammalian cells. We aim to understand how disruptions in these quality control systems contribute to the development of neurodegenerative diseases, such as Alzheimer’s, Parkinson’s, and Huntington’s disease.
Research Summary
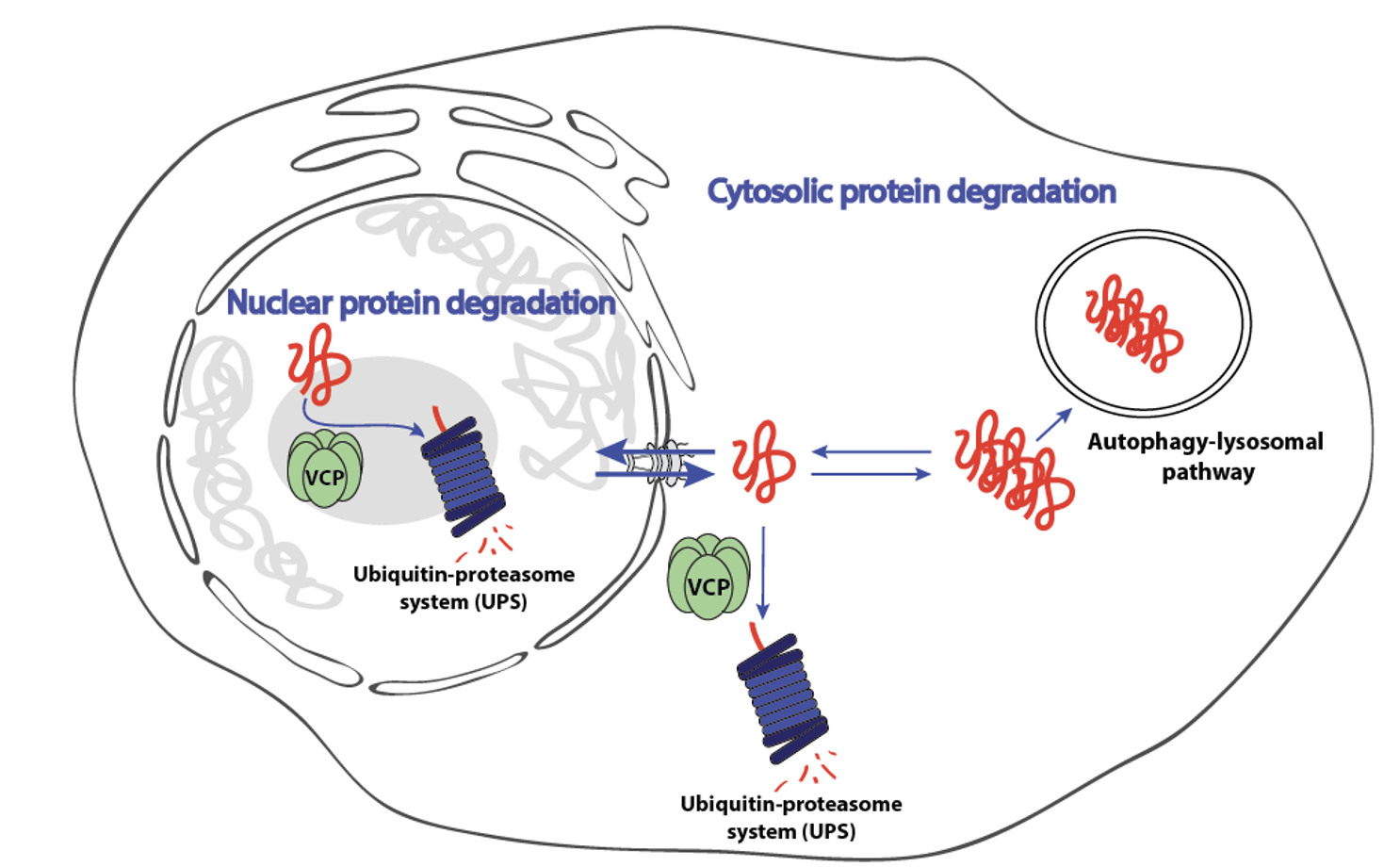
The cellular proteome is extraordinarily complex, comprising thousands of proteins that must be correctly folded, localized, and removed when damaged or no longer needed. This balance – proteostasis – is maintained by coordinated systems controlling protein synthesis, quality control, and degradation. Our laboratory focuses on the mechanisms governing protein clearance, particularly the ubiquitin-proteasome system (UPS), and how they are regulated across different cellular compartments.
Our research aims to dissect the molecular pathways that maintain nuclear protein quality control and to understand how these pathways interact with cytosolic degradation systems. We are especially interested in the nucleocytoplasmic transport of UPS components, including the unfoldase VCP/p97. Impaired communication between nuclear and cytosolic proteostasis networks may contribute to the early stages of neurodegeneration, yet the molecular underpinnings of this phenomenon remain poorly understood.
To investigate these mechanisms, we use a combination of cutting-edge molecular biology, high-throughput CRISPR/Cas9-based genetic screening, quantitative mass spectrometry, and advanced fluorescence microscopy. We apply these tools to cellular models of neurodegenerative diseases, including human iPSC-derived neurons and brain organoids. Our ultimate goal is to identify how proteostasis networks fail in disease and to uncover new therapeutic strategies aimed at restoring protein homeostasis in affected cells.
Scientific Impact
- - Identification of mechanisms regulating nuclear proteostasis and their crosstalk with cytosolic degradation pathways.
- - New insights into how alterations in protein clearance pathways contribute to neurodegenerative disease pathology.
- - Development and application of human neuronal models to study disease-associated proteostasis defects.
Future Goals
We aim to define how the ubiquitin-proteasome system functions within the nucleus and how nuclear proteostasis is integrated with broader cellular protein quality control. Our research will also explore how impairments in these systems lead to the accumulation of toxic protein aggregates – a hallmark of many neurodegenerative diseases. Ultimately, we hope to identify novel molecular targets for therapies that can restore cellular proteostasis in disease-affected tissues.
Collaborations
We actively seek interdisciplinary collaborations to bridge molecular proteostasis research with translational neuroscience. Our partnerships include experts in neurobiology, cellular imaging, proteomics, and regenerative medicine. Collaborative efforts are focused on uncovering therapeutic targets and validating findings in disease-relevant human models.
Comment
“Our goal is to understand how cells regulate the quality and clearance of proteins, especially in the nervous system. Unraveling the molecular causes of proteostasis failure in neurons may open new therapeutic avenues for currently incurable neurodegenerative diseases,” - Dr. Lidia Wróbel, Head of the Laboratory of Cellular Proteostasis.



